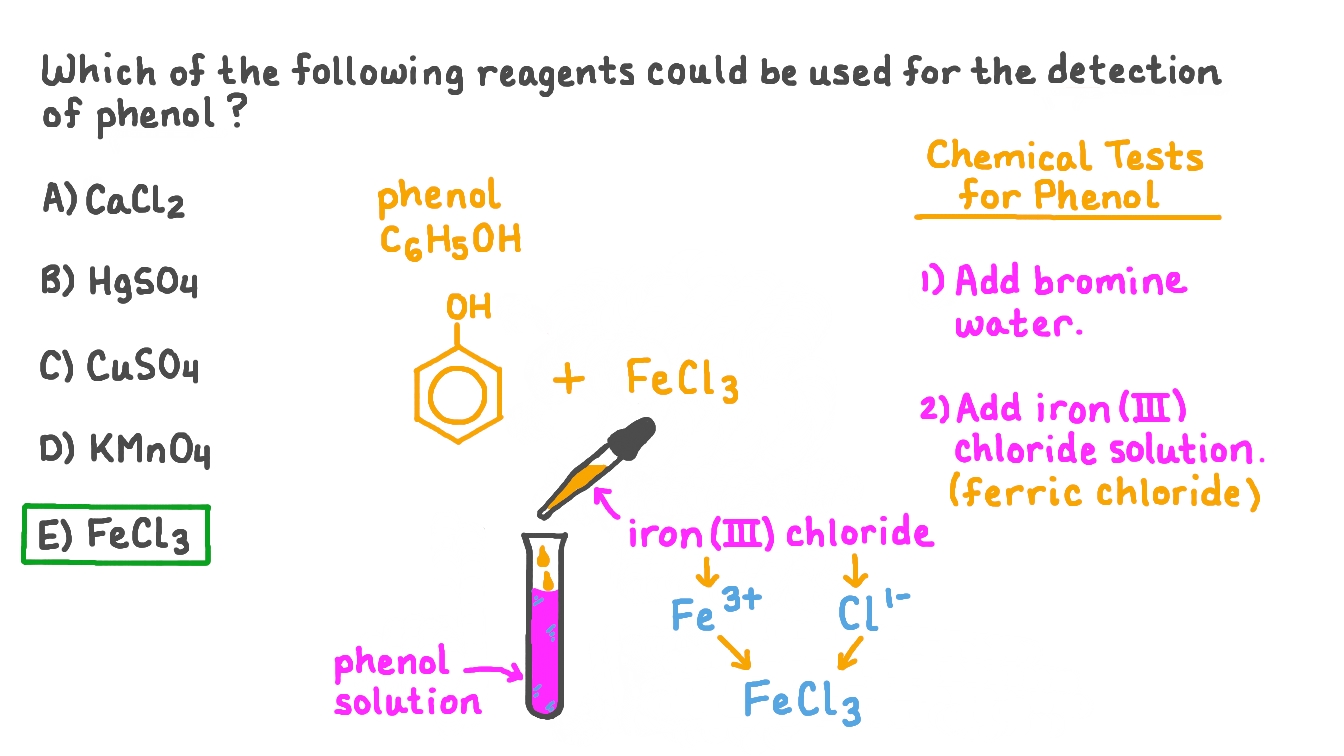
The ferric chloride test is a well-known method used in various laboratories to identify phenolic compounds. This test has become a standard procedure due to its simplicity and reliability in detecting the presence of phenols in different substances. Understanding this test can be crucial for professionals in chemistry, biology, and environmental science.
The importance of the ferric chloride test goes beyond just academic knowledge; it is also pivotal in practical applications such as pharmaceuticals, water quality testing, and material analysis. This article will delve into the intricacies of the ferric chloride test, covering its methodology, significance, and applications, ensuring that readers gain a thorough understanding of this essential chemical test.
As we explore the ferric chloride test, we will also discuss its historical context, variations, and the implications of its results. By the end of this article, readers will not only grasp the fundamental aspects of the ferric chloride test but will also appreciate its relevance in various fields.
Table of Contents
- 1. Introduction to Ferric Chloride Test
- 2. Historical Background
- 3. Methodology of the Ferric Chloride Test
- 4. Applications of the Ferric Chloride Test
- 5. Interpreting Results
- 6. Limitations of the Ferric Chloride Test
- 7. Safety Considerations
- 8. Conclusion
1. Introduction to Ferric Chloride Test
The ferric chloride test is primarily utilized for the detection of phenolic compounds, which are prevalent in various natural and synthetic substances. This test involves the reaction between ferric chloride (FeCl3) and phenolic compounds, leading to the formation of colored complexes. The color change serves as an indicator of the presence of these compounds.
Commonly used in educational settings and laboratories, the ferric chloride test is favored for its straightforward procedure and quick results. Its application ranges from organic chemistry experiments to environmental assessments, making it a versatile tool in the scientific community.
2. Historical Background
The ferric chloride test was developed in the early 19th century, marking a significant advancement in organic chemistry. Initially, this test was used in the analysis of plant extracts, wherein researchers identified various phenolic compounds responsible for specific colors in plants.
Over the years, the test has evolved and been refined, leading to more accurate and reliable results. Today, it is an essential method in both research and industrial applications.
Key Historical Milestones
- Early 1800s: Initial discoveries of phenolic compounds.
- Mid-19th century: Introduction of ferric chloride as a reagent.
- 20th century: Standardization of the test method in laboratories.
3. Methodology of the Ferric Chloride Test
The ferric chloride test is relatively simple and can be conducted using basic laboratory equipment. The methodology consists of the following steps:
- Prepare the sample solution containing the suspected phenolic compounds.
- Add a few drops of ferric chloride solution to the sample.
- Observe any color change that occurs.
Color Reactions
The color produced by the reaction can vary depending on the specific phenolic compound present:
- Yellow to brown: Indication of phenol presence.
- Green: Suggests the presence of catechol.
- Blue: Indicates the presence of certain flavonoids.
4. Applications of the Ferric Chloride Test
The ferric chloride test has numerous applications across various fields:
4.1. Environmental Testing
In environmental science, this test helps analyze water samples for phenolic pollutants, which can be harmful to aquatic life.
4.2. Pharmaceutical Industry
In pharmaceuticals, the test is vital for identifying phenolic compounds in drug formulations, ensuring quality control and safety.
4.3. Food Industry
The food industry utilizes the ferric chloride test to detect phenolic compounds in food products, which may affect flavor or safety.
5. Interpreting Results
Interpreting the results of the ferric chloride test requires an understanding of the color changes and their significance. A positive test result indicates the presence of phenolic compounds, while a negative result suggests their absence.
It is important to consider potential interferences from other substances that may cause false positives or negatives. Therefore, confirmatory tests may be necessary for accurate identification.
6. Limitations of the Ferric Chloride Test
Despite its advantages, the ferric chloride test has several limitations:
- Not all phenolic compounds react with ferric chloride.
- Color changes may be influenced by other substances present in the sample.
- Quantitative analysis is not possible using this test alone.
7. Safety Considerations
When conducting the ferric chloride test, safety precautions should be taken:
- Always wear gloves and goggles to protect against chemical exposure.
- Handle ferric chloride with care, as it can be corrosive.
- Ensure proper disposal of chemical waste according to local regulations.
8. Conclusion
In conclusion, the ferric chloride test is a valuable method for detecting phenolic compounds across various industries. Its simplicity and reliability make it a preferred choice for many laboratory applications. Understanding the methodology, applications, and limitations of this test is essential for professionals working in chemistry and related fields.
We encourage readers to engage with this topic further by leaving comments, sharing their experiences with the ferric chloride test, or exploring other related articles on our site.
Thank you for your interest, and we look forward to welcoming you back for more insightful discussions on scientific topics!
Article Recommendations
- Annual Earnings Of Katt Williams A Detailed Analysis
- Malibu Coast Fires Aftermath Recovery
- Top Music Highlights Cole Swindell Hits