Mole ratio is a fundamental concept in chemistry that plays a crucial role in understanding chemical reactions, stoichiometry, and the quantitative relationships between reactants and products. In this article, we will delve deep into the significance of mole ratio, how to calculate it, and its applications in various fields. Whether you are a student, a professional chemist, or simply someone curious about the science of molecules, this comprehensive guide will provide you with valuable insights.
The mole ratio is not just a theoretical concept; it has real-world implications that affect industries, pharmaceuticals, and even cooking. By grasping the concept of mole ratio, you can enhance your understanding of the chemical processes that occur around you. Furthermore, this knowledge can empower you to make informed decisions in your daily life, especially when it comes to recipes, chemical safety, and environmental considerations.
Throughout this article, we will explore the definition of mole ratio, step-by-step calculations, and practical examples that highlight its importance. Additionally, we will address common questions and misconceptions surrounding mole ratio, ensuring that you leave with a well-rounded grasp of the topic. Let’s embark on this journey to explore the fascinating world of mole ratios!
Table of Contents
- Definition of Mole Ratio
- Importance of Mole Ratio in Chemistry
- How to Calculate Mole Ratio
- Examples of Mole Ratio in Chemical Reactions
- Applications of Mole Ratio
- Common Misconceptions About Mole Ratio
- Practical Aspects of Mole Ratio in Everyday Life
- Conclusion
Definition of Mole Ratio
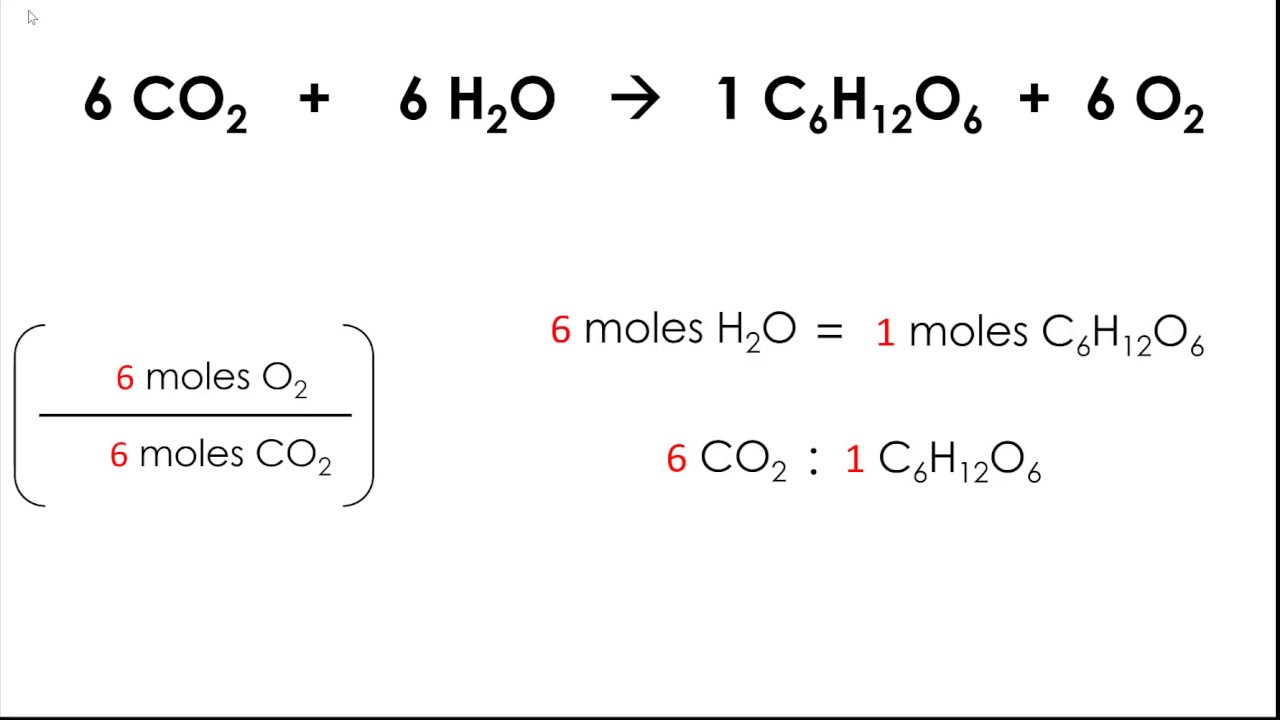
Mole ratio is defined as the ratio of the number of moles of one substance to the number of moles of another substance in a chemical reaction. It is derived from the coefficients of the balanced chemical equation, which indicate the proportions of reactants and products involved in the reaction. For example, in the reaction:
2 H₂ + O₂ → 2 H₂O
The mole ratio of hydrogen (H₂) to oxygen (O₂) is 2:1, meaning that two moles of hydrogen react with one mole of oxygen to produce water.
Importance of Mole Ratio in Chemistry
Mole ratios are essential in various aspects of chemistry, including:
- Stoichiometry: Mole ratios allow chemists to calculate the amounts of reactants needed or products formed in a reaction.
- Balancing Equations: Understanding mole ratios helps in balancing chemical equations accurately.
- Predicting Yields: Mole ratios aid in predicting the theoretical yield of a reaction based on reactant quantities.
How to Calculate Mole Ratio
Calculating mole ratios involves a few straightforward steps:
- Write the balanced chemical equation: Ensure that the equation is balanced with correct coefficients.
- Identify the substances: Determine which substances you want to compare in terms of their mole ratio.
- Use the coefficients: The coefficients in the balanced equation provide the necessary mole ratios. For example, in the equation above, the mole ratio is derived from the coefficients 2 (for H₂) and 1 (for O₂).
Examples of Mole Ratio in Chemical Reactions
Let’s look at a couple of examples to illustrate how mole ratios work:
Example 1: Combustion of Methane
In the combustion of methane (CH₄):
CH₄ + 2 O₂ → CO₂ + 2 H₂O
The mole ratio of methane to oxygen is 1:2, indicating that one mole of methane reacts with two moles of oxygen.
Example 2: Synthesis of Ammonia
In the synthesis of ammonia (NH₃):
N₂ + 3 H₂ → 2 NH₃
The mole ratio of nitrogen to hydrogen is 1:3, meaning one mole of nitrogen reacts with three moles of hydrogen to produce two moles of ammonia.
Applications of Mole Ratio
Mole ratios have numerous applications across various fields, including:
- Chemical Manufacturing: Industries rely on mole ratios to optimize the production of chemicals efficiently.
- Pharmaceuticals: Accurate mole ratios are critical in drug formulation and dosage calculations.
- Environmental Science: Mole ratios help in assessing pollutant levels and chemical reactions in ecosystems.
Common Misconceptions About Mole Ratio
There are several misconceptions surrounding mole ratios, including:
- All reactions have a 1:1 ratio: This is incorrect, as reactions can have various mole ratios depending on the reactants and products.
- Mole ratios are only relevant to gases: Mole ratios apply to all states of matter—solids, liquids, and gases.
Practical Aspects of Mole Ratio in Everyday Life
Mole ratios can also be observed in everyday situations, such as:
- Cooking: Recipes often require specific ratios of ingredients for proper flavor and texture.
- Cleaning Products: Understanding the ratios of chemicals in cleaning products can enhance their effectiveness.
- Gardening: The ratio of nutrients in fertilizers can impact plant growth and health.
Conclusion
In conclusion, the mole ratio is a vital concept in chemistry that underpins many scientific and practical applications. Understanding mole ratios enables chemists to conduct accurate calculations and predictions, ultimately leading to advancements in various fields. Whether you are balancing equations, predicting yields, or even cooking, the knowledge of mole ratios can enhance your understanding and effectiveness. We encourage you to explore further and consider how mole ratios impact your life and the world around you!
If you have any questions or thoughts about mole ratios, feel free to leave a comment below. Share this article with friends or colleagues who might find it helpful, and don’t hesitate to explore more articles on our site for additional insights!
Article Recommendations
- Aj Johnson Contracts Top Projects Expertise
- Megan Fox And Shia Labeouf A Hollywood Romance Revisited
- Megan Fox Mgk Drama Latest Updates Fallout